Abstract
Background
Cardiac resynchronisation therapy (CRT) is a well-established form of treatment for patients with heart failure and cardiac dyssynchrony. There are two different types of CRT devices: the biventricular pacemaker (CRT-P) and the biventricular defibrillator (CRT-D). The latter is more complex but also more expensive. For the majority of patients who are eligible for CRT, both devices are appropriate according to current guidelines. The purpose of this study was to conduct a cost-utility analysis for CRT-D compared to CRT-P from a German payer’s perspective.
Methods
A cohort Markov-model was developed to assess average costs and quality-adjusted life-years (QALY) for CRT-D and CRT-P. The model consisted of six stages: one for the device implementation, one for the absorbing state death, and two stages (“Stable” and “Hospital”) for either a CRT device or medical therapy. The time horizon was 20 years. Deterministic and probabilistic sensitivity analyses and scenario analyses were conducted.
Results
The incremental cost-effectiveness ratio (ICER) of CRT-D compared with CRT-P was €24,659 per additional QALY gained. In deterministic sensitivity analysis, the survival advantage of CRT-D to CRT-P was the most influential input parameter. In the probabilistic sensitivity analysis 96% of the simulated cases were more effective but also more costly.
Conclusions
Therapy with CRT-D compared to CRT-P resulted in an additional gain of QALYs, but was more expensive. In addition, the ICER was subject to uncertainty, especially due to the uncertainty in the survival benefit. A randomised controlled trial and subgroup analyses would be desirable to further inform decision making.
Similar content being viewed by others
Treatment with the biventricular pacemaker (CRT-P) is less expensive than treatment with the biventricular defibrillator (CRT-D). But treatment with CRT-D resulted in a higher expected median survival. |
The cost difference between CRT-D and CRT-P is largely influenced by device costs, more frequent hospitalisations and shorter device longevity. |
The uncertainty in the cost-effectiveness ratio is mainly driven by uncertainty in the survival benefit of CRT-D compared to CRT-P. |
1 Background
Heart failure is one of the leading causes of death in Germany [1]. It reduces survival and impairs quality of life [2,3,4]. Healthcare resource utilisation is high in heart failure—especially due to hospitalisations [5]. The prevalence of heart failure in Germany ranges between 2% and 4%, and rises with age [6, 7]. Cardiac resynchronisation therapy (CRT) is indicated, with the highest recommendation level for patients with symptomatic heart failure in New York Heart Association (NYHA) classes II–IV, reduced ejection fraction ≤ 35% and broad QRS complex, according to the current corresponding European guideline [8].
CRT is a well-established form of treatment that relies on two different treatment options: the biventricular pacemaker (CRT-P) and the cardiac biventricular defibrillator (CRT-D). The additional defibrillator is intended to protect patients from sudden cardiac death. However, CRT-D devices are more complex and costly, with a higher hospitalisation rate due to lead failure, infections or inappropriate shocks, which impairs quality of life [9, 10]. In Germany, 21,479 CRT procedures were performed in 2015, of which about 80% were CRT-D implementations. The relative share of CRT-D on all CRT devices is considerably higher in Germany compared to other European countries [2].
Several RCTs have shown that patients with CRT devices have significantly better outcomes compared to patients solely treated with optimal medical therapy (OMT) or an implantable cardioverter defibrillator [11,12,13]. However, there has been no sufficiently powered head-to-head trial of CRT-D and CRT-P to date. For the majority of patients who are eligible for CRT, both devices are appropriate according to the current guideline [8].
Two studies conducted a health economic evaluation for the German healthcare system, either for CRT-P versus OMT [14] or for CRT-D versus OMT [15]. Therefore, this study aimed to evaluate cost-effectiveness of CRT-D versus CRT-P by an indirect comparison from a German statutory health insurance (SHI) perspective. Since survival is the crucial parameter in this evaluation, a long-term perspective was applied by extrapolating the survival of Kaplan–Meier curves.
2 Methods
A Markov-model was developed to analyse the cost-effectiveness of CRT-D + OMT compared to CRT-P + OMT. To perform a comprehensive analysis the results of CRT-P + OMT compared to OMT are reported as well, as OMT is the low-cost alternative to treat this patient cohort. The model outcomes were quality-adjusted life-years (QALYs) and costs from a German SHI payer’s perspective.
Heart failure is a chronic disease and most common in older people; therefore extrapolation beyond the follow-up was necessary. After 20 years, the model predicted that 13% of CRT-D patients, 10% of CRT-P patients and 0% of OMT patients were still alive. For this reason, a time horizon of 20 years was chosen because a longer life model would add unnecessary uncertainty and the major health and economic outcomes could be captured. In addition, the model converged from that time onwards. Model results for 9 years, the maximum follow-up time of CARE-HF and for 15 years are reported as well. The cycle length was 1 month. The model was conducted for three identical and homogeneous cohorts, differing only in the three treatment strategies: (1) CRT-P + OMT, (2) CRT-D + OMT or (3) OMT. The model was run for a cohort of 1000 hypothetical patients for each strategy. Using a cohort simulation, expected costs and expected survival were calculated [16]. Costs and QALYs were discounted by 3% per year [17]. The outcomes were used to calculate the incremental cost-effectiveness ratio (ICER), which indicates the amount to be spent for an additional QALY. The model was set up in “R” [18] with the package “heemod” [19].
2.1 Target Population
In the European guideline, CRT is recommended for patients with reduced ejection fraction ≤ 35%, broad QRS complex and symptomatic heart failure in NYHA classes II–IV despite OMT [8]. Three treatment options are available for this patient group: OMT, CRT-P and CRT-D. The patients face three different causes of death: heart failure death, sudden cardiac death and non-cardiac death. The additional defibrillator of a CRT-D device should reduce sudden cardiac death. There are two RCTs that cover this patient collective: COMPANION [20] and CARE-HF [21]. The CARE-HF trial was chosen for the analysis of survival because it comprises the longest follow-up of a patient heart-failure collective due to left ventricular systolic dysfunction and cardiac dyssynchrony, which is the relevant patient cohort for researching cost-effectiveness of CRT-D compared to CRT-P. The initial age (66 years) of the hypothetical cohort was set according to the median age of CARE-HF patients at baseline.
2.2 Model Structure
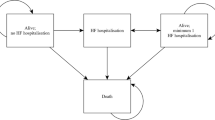
The model consists of six Markov-states (Fig. 1). All patients entered the model via the implementation surgery, which could either be successful or fail. The main part was divided into a CRT section and an OMT section. In case the implementation surgery failed, patients were solely treated with OMT. Each subsection of the model comprised a Markov state “Stable” and a Markov state “Hospital”, which included hospitalisations due to heart failure, lead failure, a device infection or ventricular arrhythmia. The distribution of NYHA classes over time was taken from Colquitt et al. [11] (see Table 1). The absorbing state was all-cause death. The possibility of device upgrades was excluded. The same events could occur in the OMT section, except for hospitalisations due to device malfunctions or infections.
2.3 Model Parameters
The model parameters for the chosen model structure consisted of the mortality risk, the probability of adverse events, costs that occur for specific events and health-related quality-of-life (HRQoL) values for the health states in the model. The main model was deterministic. Mortality was the crucial parameter in this modelling. To date there has been no randomised, controlled trial (RCT) that directly compared CRT-D to CRT-P, but many meta-analyses comparing CRT devices have already been performed. Therefore, meta-analyses have been systematically searched via PubMed to investigate the effect of the devices on mortality. We chose the analysis of Woods et al. [12] because they were the only ones who performed a network meta-analysis of RCTs with individual patient data of CRT-D, CRT-P, OMT and ICD trials. More recent meta-analyses such as Barra et al. [13] have not included other relevant RCTs.
Input parameters for the transition probabilities and device longevity calculations are based on single RCTs used by six meta-analyses [11,12,13, 22,23,24]. Two additional references [9, 25] were found by literature search via PubMed. With the exception of one observational study [9], only RCTs were used to compute transition probabilities, as these generally have a higher level of evidence. The probabilities were transformed to the cycle length of 1 month [16] and were pooled with a random-effects model [26] (see Table 2).
One-way deterministic sensitivity analyses were conducted (see Table 2). For the probabilistic sensitivity analysis, a Monte Carlo simulation with 10,000 iterations was applied, where the values of the input parameters were assumed to follow certain probability distributions. Based on the recommendations by the ISPOR guideline, distributions for the input parameters were chosen [27]. The input parameters for all three treatment strategies are listed in Table 2.
2.3.1 Effectiveness Data
To estimate survival, the Kaplan–Meier curves for all-cause mortality in the CARE-HF [28, 29] trial for patients with either CRT-P or OMT were digitalised with DigitizeIt [30]. For OMT the publication with the shorter follow-up was chosen because in the subsequent publication a lot of treatment cross-overs were reported. In the CARE-HF study CRT-P was compared to OMT with a maximum follow-up of 9 years. The individual patient data were reconstructed using the R-code by Guyot et al. [31]. We plotted cumulative hazard functions for OMT and CRT-P. In both plots the hazard is monotonically increasing, which indicates a distribution that enables increasing hazards. Afterwards parametric survival curves were fitted (exponential, Weibull, Gompertz, gamma, log-logistic, lognormal). The differences in the Akaike Information Criterion (AIC) indicated a similarly good fit for all curves, except maybe for lognormal and log-logistic. Thus, the parametric survival distributions were selected according to visual conformity to the original Kaplan–Meier survival curve and plausibility in the long run. A Gompertz distribution was chosen for CRT-P and for OMT.
To construct a survival curve for CRT-D, a hazard ratio of 0.81 was applied to the parametric survival curve of CRT-P in order to derive device-dependent mortality rates. This value for the hazard ratio [95% confidence interval (CI) 0.67–0.99] was reported by Woods et al. in an individual patient data network meta-analysis [12], which incorporates the major RCTs for patients with heart failure and reduced ejection fraction who received an OMT, ICD, CRT-P or CRT-D, and is therefore considered the highest available evidence. In total, the meta-analysis included 12,638 patients with 2422 deaths. Mean follow-up was 2.5 years (range 0–7.5 years). For ten trials individual patient data were available. An unadjusted network meta-analysis was performed to determine the overall efficacy of the devices throughout all RCTs. An adjusted analysis was performed for different baseline characteristics to evaluate whether these subgroups experienced different treatment effects. In line with Mealing et al. [32], we assumed that the hazard ratio started to increase after 7.5 years, which was the maximum follow-up in the meta-analysis by Woods et al. [12], until it was 1 after 20 years. The observed survival curves for OMT and CRT-P were based on data from patients aged on average 66 years and the parametric survival curves were then extrapolated over the model time horizon (maximum follow-up 9 years, extrapolation for 20 years). The extrapolation of survival curves over such a long time period is subject to uncertainty. Thus, three scenario analyses were run for that input parameter.
The reported device longevity for CRT-P and CRT-D varied between the cost-effectiveness studies. Therefore, the median device longevity was taken from a National Institute for Health and Care Excellence (NICE) report calculated from approximately 40,000 implementations from 2000 to 2011 [33]. Lower and higher device longevity estimates were taken from cost-effectiveness studies for the sensitivity analysis [30,31,32] (see Table 2). In the case of battery depletion the whole CRT system has to be replaced.
2.3.2 Quality of Life
Beside the importance of survival, heart failure is characterised by decreased quality of life and frequent hospitalisations, which were assumed to also impair quality of life. Quality of life decreases with progress of heart failure. Boczor estimated HRQoL weights for German patients with chronic heart failure (n = 3387) with the EQ-5D-5L questionnaire [34]. HRQoL weights were reported for each NYHA class (range 0.834–0.564). We assumed that patients in Markov-state “Hospital” have a reduced quality of life because hospitalisation itself can negatively affect a patient’s quality of life and, moreover, a worsened health condition has led to hospitalisation that has affected the quality of life as well. HRQoL decrements for a hospitalisation were taken from Griffiths et al. [35] according to the severity of the NYHA class (range − 0.07 to − 0.21). We applied values from other CRT cost-effectiveness studies from 0.93 to 0.6 [23, 36] and 0.815–0.44 [23, 37] as upper and lower bounds within the deterministic sensitivity analysis.
2.3.3 Costs
All costs associated with hospitalisation were calculated using the reimbursement catalogue for hospital admissions (G-DRG flat rate catalogue) [38]. Each hospitalisation could be matched to one or more diagnoses-related group (DRG) codes and each DRG code is graded in ascending case severity. The costs for a hospitalisation event were estimated from these different DRG codes and their severity grades. This was achieved by weighting the DRGs with their distribution of case severities from the G-DRG Report Browser 2018, depending on ICD and OPS (operation and procedure keys) codes [39].
For instance inpatient treatment for heart failure is grouped into four DRGs (F62A: €10,894.26; F62B: €8,058.05; F62C: €2,943.74; F62D: €794.01). Weighting these DRGs with the G-DRG-Report Browser 2018 resulted in a hospitalisation cost for heart failure of €2,926.43. All other costs related to the management of heart failure such as outpatient, rehabilitation and medication costs subject to the NYHA class were derived from Biermann et al. [40] and inflated to 2018 prices (GDP inflation rate 1.38 [41]).
Costs incurred as a result of the implementation surgery were not considered because in Germany all additional hospital stays within 30 days are covered by one single DRG (case consolidation) and thus do not differ between CRT-D and CRT-P [42]. In the base-case analysis, we did not consider future costs that are not related to heart failure.
3 Results
The predicted median survival of CRT-D was 7.8 years and of CRT-P was 6.5 years (see Fig. 2). The median survival with OMT was 4.1 years. Treatment of patients with CRT-D caused an average cost of €32,447, treatment with CRT-P an average cost of €18,502 and with OMT €5,472. Hence, CRT-D compared to CRT-P resulted in €13,945 incremental costs per patient. The incremental cost of CRT-P compared to OMT was €13,029. When comparing CRT-D to CRT-P, 0.57 incremental QALYs were gained over the time horizon. Comparing CRT-P to OMT, 2.23 incremental QALYs were gained. The ICER was €24,659 per additional QALY for CRT-D compared to CRT-P and €5,837 for CRT-P versus to OMT respectively. Table 3 shows the disaggregated results for CRT-D to CRT-P and CRT-P to OMT for different model runtimes.
3.1 Sensitivity Analysis
The top ten most influential input parameters on the ICER are depicted in a tornado diagram in Fig. 3a. The survival benefit (hazard ratio) of CRT-D compared to CRT-P was the input parameter with the most influence on the ICER. As an illustration of how strongly the survival benefit influenced the ICER, Fig. 3b shows the ICER as a function of the survival benefit, using the CI of the hazard ratio (0.67–0.99) as limits. Even small changes in the survival benefit had a major influence. Other influential parameters were the device cost of a CRT-D, the device longevity for CRT-D and probability of the implementation success. In further sensitivity analyses other HRQoL estimates did not greatly alter the model result. With regard to different time horizons, the ICER per additional QALY for 9 years was €41,020 and for 15 years €27,016. The results of the Monte Carlo simulation are presented in the cost-effectiveness plane (Fig. 3c). In total, 96% of all simulated cases were in the north-east quadrant with positive incremental costs and positive incremental QALYs. In 4% of the simulated cases CRT-P was dominant. The average ICER of the Monte Carlo simulation was €22,477 for an additional QALY. In the probabilistic sensitivity analysis, not the costs for hospitalisations were varied, but the distributions of the DRG severity. In Fig. 3d the cost-effectives acceptability curve (CEAC) is illustrated. It displays the probability of being cost-effective at a given willingness-to-pay.
Results of sensitivity analysis of the cost-utility analysis. a Tornado diagram; b incremental cost-effectiveness ratio as a function of the hazard ratio; c Monte-Carlo simulation; d cost-effectiveness acceptability curve. CRT-D cardiac biventricular defibrillator, CRT-P biventricular pacemaker, ICER incremental cost-effectiveness ratio
3.2 Scenario Analysis
Five scenario analyses were conducted for the cost-utility analysis of CRT-D compared to CRT-P. First, life-years (LY) were chosen as an outcome parameter. The ICER was €18,945 per additional LY. Second, to capture overall digitalisation and parametrisation uncertainty, the parametrisation of the CARE-HF trial by Colquitt et al. [11] was used to model survival. Using these data the ICER yielded €21,597 per additional QALY. Since the CARE-HF study is older it can be assumed that the treatment of heart failure has changed over time. Third, to account for possible treatment changes, the Kaplan–Meier curve for CRT-P of a long-term observational study was parametrised [43] and for CRT-D the hazard ratio was applied. This scenario yielded an ICER of €22,073 per additional QALY. This is just a rough estimate because the digitalised Kaplan–Meier curve was a little imprecise (only 550 of 580 deaths could be reconstructed). The estimated median survival varied only slightly between the different parametrisations. Corrao et al. [44] estimated the probability of death within 30 days of hospital discharge (0.047). As a fourth scenario analysis, this estimate was used to correct the probability of hospital mortality upwards for the period after the maximum follow-up. The ICER was €26,006 per additional QALY. Fifth, we calculated the ICER for additional future costs that were unrelated to heart failure. Applying an additional €7,275 per year for individuals aged 65–84 years and €16,616 for individuals over 85 years [45] resulted in an ICER of €34,460 per additional QALY.
4 Discussion
Applying a cohort Markov-modelling approach, we aimed to assess evidence on the cost-effectiveness of the CRT-D compared to CRT-P from a payer’s perspective. The development of the model was supported by systematic reviews for modelling heart failure, in particular CRT [42,43,44], various cost-effectiveness CRT studies [11, 36, 37], as well as by feedback from a cardiologist.
In the base case, therapy with CRT-D was more effective but also more costly. For the German healthcare system there was already one study for CRT-D compared to OMT [15] and one study for CRT-P compared to OMT [14]. Both CRTs were found to be more effective than OMT. Our study added the indirect comparison of CRT-D to CRT-P. In addition, we also compared CRT-P to OMT. In the comparison of CRT-D to CRT-P, the resulting ICER was €24,659 per additional QALY and €5,837 for CRT-P to OMT, respectively.
Our estimated ICER is lower than the ICERs reported in other studies investigating the cost-effectiveness of CRT-D to CRT-P. The range of costs per additional QALY reported in the literature is €30,447–€56,719 in 2014 prices [24]. Most of the studies are based on survival data from COMPANION or CARE-HF. However, the other analyses differ significantly from ours in utility weights, CRT device runtime, model time horizon, and the hazard ratio used. ICER differences could also be attributed to modelling disparities. A further reason for the lower ICER could be decreased costs for CRT devices over time or the reduced price difference between the CRT devices. The British HTA by Colquitt et al. [11] reported an ICER of €30,420 per additional QALY, which is quite similar to our one [24].
In Germany no official threshold exists for the assessment of cost-effectiveness. If one follows the result of a recent study that surveyed individuals in Germany about their willingness to pay (WTP) per additional QALY, CRT-D would not be cost-effective. In the study, the WTP ranged from €8580 to €18,420 per additional QALY [46]. CRT-P, on the contrary, would be cost-effective compared to OMT in relation to the specific WTP per QALY. According to the NICE guideline, the ICER for an additional QALY would be cost-effective [range €23,600–€35,400; 1.18 as exchange rate (27 March 2019)] [47]. The CEAC (Fig. 3d) can be interpreted as the probability of being cost-effective at a given threshold from a payer’s perspective. Taking the upper boundary of the NICE threshold as a hypothetical WTP, CRT-D would be cost-effective with a probability of 77% for an additional QALY.
With respect to the sensitivity analysis the model was robust, only 4% of the probabilistic ICERs were in the North-West quadrant. However, the model results have sensitively responded to changes in the survival benefit. It had by far the strongest influence on the model results. There is an ongoing debate about the additional value of the defibrillator in science [48, 49]. For instance, a reduction of sudden cardiac death as a result of improved medical therapy such as sacubitril/valsartan or improved utilisation of beta blockers, ACE inhibitors and mineralocortocioid antagonists [50], would be expected to decrease the survival benefit of CRT-D and thereby increase the ICER. In addition, Shen et al. [51] reported that the rate of sudden cardiac death in heart failure studies decreases over time. Furthermore, in patients who survived the first 5 years after implementation, the risk of sudden cardiac death was low, and the type of device was not a significant predictor for survival [48]. For this reason, the survival benefit of CRT-D decreased steadily after the maximum follow-up time in the meta-analysis [12]. A reduced survival benefit could be seen as critical in terms of harms aligned with CRT-D devices, such as device malfunctions, increased complication risk or impaired quality of life due to inadequate shocks [52, 53, 54].
Discussions pertaining the impact of survival benefits on cost-utility of CRT-D must be approached with caution, as the model input data for survival comes with some degree of uncertainty. While we acknowledge that we have used the best available hazard ratio for the survival benefit, there are some restrictions. First, the survival curves of CRT-P and OMT have been extrapolated to a long term scenario. Second, since no direct comparison of CRT-D to CRT-P was available in an RCT, the death probability of CRT-D was estimated with a hazard ratio applied to the survival curve of CRT-P.
Another influential input parameter was the device longevity. Device life and device changing costs are interconnected. With higher battery capacity, the high device changing costs would be incurred less frequently. Technological progress in battery capacity would change the ICER in favour of CRT-D. There are some studies on the longevity of CRT-D devices [55, 56], but there are no studies explicitly researching for CRT-P device longevity [2], and Colquitt et al. [11] noted that the estimates that were reported by NICE [33] could be overestimated. The ICER for a model horizon of 15 years was comparable to the base-case ICER, but if the model horizon was only 9 years, the survival advantage was not so pronounced.
The study has several limitations. First, as already mentioned, the input parameters were derived from older studies. Thus, evidence may be outdated considering the significant progress that has been made in treatment of heart failure patients. To control for this effect in the scenario analysis, the Kaplan–Meier curves of the recent 16-year-long observational study by Leyva et al. [43] were used to model survival, which did not change the results substantially. Second, hospitalisation costs were modelled accurately while outpatient and medication costs were approximated, and costs of sacubitril/valsartan were not included. However, this bias is assumed to be negligible because these parts of the costs were comparatively low. Third, there are more complex approaches to model heart failure that take more possible events into account, but the data basis is partly not sufficient and moreover the survival benefit superimposes most input parameters. Fourth, the probability of hospitalisation or death was not based on time-dependent variables like previous hospitalisations, since no data were available. Fifth, information on NYHA class changes was only available until the 19th month. The cost of monthly NYHA management and utilities depends on NYHA classes. We conducted scenario analyses, assuming the same NYHA class distribution for OMT and CRT for the remaining runtime. Model results were not sensitive to changes in this parameter.
In contrast to Yao et al. [37] and Colquitt et al. [11], this modelling approach did not distinguish between different mortality sub-classifications, because otherwise the hazard ratio by Woods et al. [12] would not have been sufficient to inform the model. The hazard ratio is taken from a patient data network meta-analysis of RCT-CRT studies, and was therefore assumed to be the best available evidence. Moreover, such a division of mortality could be inaccurate [12], and Cleland et al. [28] only reported number at risk in the Kaplan–Meier curves for all-cause death, which was needed to digitalise the curves with the highest accuracy.
A strength of the modelling is the use of pooled-effect estimators for the input parameters wherever feasible. To check for inaccuracy in the digitalisation process of the Kaplan–Meier curves, the parametrisation of Colquitt et al. [11] was used, which did not change the ICER.
5 Conclusions and Future Research
A literature-based cohort Markov-model was developed to examine cost-effectiveness for CRT-D to CRT-P from a payer’s perspective in Germany. CRT-D compared to CRT-P was more effective but also more costly, yielding an ICER of €24,659 for an additional QALY and an ICER of €18,945 for an additional life-year. Future technological changes in device longevity or lower device costs for CRT-D could reduce the ICER. Although results have to be seen in the context of uncertainty especially concerning the survival benefit, our sensitivity analysis shows that our model results were robust. For a reduction of uncertainty a direct comparison under RCT conditions with a longer follow-up would be desirable. It could provide the necessary data to perform subgroup analyses at the patient level to weigh the advantages and disadvantages more properly.
Data Availability
All data used for this study are public and referenced throughout the manuscript. The Excel sheet and the R code are available from the corresponding author on reasonable request.
References
Statistisches Bundesamt. Krankheitskosten, Sterbefälle insgesamt 2016 [Internet]. www.destatis.de. 2016. https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Gesundheit/Todesursachen/Tabellen/sterbefaelle-herz-kreislauf-erkrankungen-insgesamt.html. Accessed 28 Mar 2019.
Deutsche Herzstiftung e.V. Deutscher Herzbericht 2016. Sektorenübergreifende Versorgungsanalyse zur Kardiologie, Herzchirurgie und Kinderherzmedizin in Deutschland. Frankfurt am Main; 2016.
Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–46.
Christ M, Störk S, Dörr M, Heppner HJ, Müller C, Wachter R, et al. Heart failure epidemiology 2000–2013: insights from the German Federal Health Monitoring System: heart failure in Germany (2000–2013). Eur J Heart Fail. 2016;18:1009–18.
Neumann T, Biermann J, Neumann A, Wasem J, Ertl G, Dietz R, et al. Heart failure: the Commonest Reason for Hospital Admission in Germany. Deutsches Aerzteblatt Online. 2009;106:269–75.
Störk S, Handrock R, Jacob J, Walker J, Calado F, Lahoz R, et al. Epidemiology of heart failure in Germany: a retrospective database study. Clin Res Cardiol. 2017;106:913–22.
Ohlmeier C, Mikolajczyk R, Frick J, Prütz F, Haverkamp W, Garbe E. Incidence, prevalence and 1-year all-cause mortality of heart failure in Germany: a study based on electronic healthcare data of more than six million persons. Clin Res Cardiol. 2015;104:688–96.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200.
Barra S, Providência R, Boveda S, Duehmke R, Narayanan K, Chow AW, et al. Device complications with addition of defibrillation to cardiac resynchronisation therapy for primary prevention. Heart. 2018;104:1529–35.
Sears SF, Hauf JD, Kirian K, Hazelton G, Conti JB. Posttraumatic stress and the implantable cardioverter-defibrillator patient: what the electrophysiologist needs to know. Circ Arrhythmia Electrophysiol. 2011;4:242–50.
Colquitt JL, Mendes D, Clegg AJ, Harris P, Cooper K, Picot J, et al. Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure: systematic review and economic evaluation. Health Technol Assess. 2014;18:1–560.
Woods B, Hawkins N, Mealing S, Sutton A, Abraham WT, Beshai JF, et al. Individual patient data network meta-analysis of mortality effects of implantable cardiac devices. Heart. 2015;101:1800–6.
Barra S, Providência R, Duehmke R, Boveda S, Begley D, Grace A, et al. Cause-of-death analysis in patients with cardiac resynchronization therapy with or without a defibrillator: a systematic review and proportional meta-analysis. EP Europace. 2018;20:481–91.
Banz K. Cardiac resynchronization therapy (CRT) in heart failure—a model to assess the economic value of this new medical technology. Value Health. 2005;8:128–39.
Aidelsburger P, Grabein K, Klauss V, Wasem J. Cost-effectiveness of cardiac resynchronization therapy in combination with an implantable cardioverter defibrillator (CRT-D) for the treatment of chronic heart failure from a German health care system perspective. Clin Res Cardiol. 2008;97:89–97.
Briggs AH, Claxton K, Sculpher MJ. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWIG). Allgemeine Methoden: Version 5.0 [Internet]. Köln; 2017. https://www.iqwig.de/download/Allgemeine-Methoden_Version-5-0.pdf. Accessed 10 Apr 2019.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. https://www.R-project.org/.
Filipović-Pierucci A, Zarca K, Durand-Zaleski I. Markov Models for Health Economic Evaluations: The R Package heemod. arXiv:170203252 [stat] [Internet]. 2017. http://arxiv.org/abs/1702.03252. Accessed 9 Jan 2019.
Bristow MR, Krueger S, Carson P, White BG. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;11:2140–50.
Cleland JGF, Erdmann E, Kappenberger L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;11:1539–49.
Di Tanna GL, Bychenkova A, O’Neill F, Wirtz HS, Miller P, Ó Hartaigh B, et al. Evaluating cost-effectiveness models for pharmacologic interventions in adults with heart failure: a systematic literature review. PharmacoEconomics [Internet]. 2018. http://link.springer.com/10.1007/s40273-018-0755-x. Accessed 15 Jan 2019.
Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al. The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model. Health Technology Assessment [Internet]. 2007. https://www.journalslibrary.nihr.ac.uk/hta/hta11470/. Accessed 14 Oct 2018.
Tomini F, Prinzen F, van Asselt ADI. A review of economic evaluation models for cardiac resynchronization therapy with implantable cardioverter defibrillators in patients with heart failure. Eur J Health Econ. 2016;17:1159–72.
Ruwald MH, Solomon SD, Foster E, Kutyifa V, Ruwald A-C, Sherazi S, et al. Left ventricular ejection fraction normalization in cardiac resynchronization therapy and risk of ventricular arrhythmias and clinical outcomes: results from the multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy (MADIT–CRT) trial. Circulation. 2014;130:2278–86.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48.
Briggs AH, Weinstein MC, Fenwick EAL, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM modeling good research practices task force-6. Value Health. 2012;15:835–42.
Cleland JGF, Daubert J-C, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase]. Eur Heart J. 2006;27:1928–32.
Cleland JGF, Freemantle N, Erdmann E, Gras D, Kappenberger L, Tavazzi L, et al. Long-term mortality with cardiac resynchronization therapy in the Cardiac Resynchronization-Heart Failure (CARE-HF) trial. Eur J Heart Fail. 2012;14:628–34.
Bormann I. DigitizeIt—Plot Digitizer Software Installation and Unistallation [Internet]. https://www.digitizeit.de/contact.html. Accessed 27 May 2019.
Guyot P, Ades A, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol [Internet]. 2012. https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/1471-2288-12-9. Accessed 13 Mar 2019.
Mealing S, Woods B, Hawkins N, Cowie MR, Plummer CJ, Abraham WT, et al. Cost-effectiveness of implantable cardiac devices in patients with systolic heart failure. Heart. 2016;102:1742–9.
National Institute for Health and Care Excellence. Final appraisal determination. Implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure (review of TA95 and TA120) [Internet]. London: National Institute for Health and Care Excellence; 2014, p. 1–75. https://www.nice.org.uk/guidance/ta314/documents/arrythmias-icds-heart-failure-cardiac-resynchronisation-fad-document2. Accessed 15 June 2019.
Boczor S. Eine Validierung des deutschsprachigen Fragebogens EQ-5D-5L für Patientinnen und Patienten mit Herzinsuffizienz in Deutschland. [Hamburg]: Universität Hamburg; 2017.
Griffiths A, Paracha N, Davies A, Branscombe N, Cowie MR, Sculpher M. Analyzing health-related quality of life data to estimate parameters for cost-effectiveness models: an example using longitudinal EQ-5D data from the SHIFT randomized controlled trial. Adv Ther. 2017;34:753–64.
Bertoldi EG, Rohde LE, Zimerman LI, Pimentel M, Polanczyk CA. Cost-effectiveness of cardiac resynchronization therapy in patients with heart failure: the perspective of a middle-income country’s public health system. Int J Cardiol. 2013;163:309–15.
Yao G, Freemantle N, Calvert MJ, Bryan S, Daubert J-C, Cleland JGF. The long-term cost-effectiveness of cardiac resynchronization therapy with or without an implantable cardioverter-defibrillator. Eur Heart J. 2006;28:42–51.
Institut für das Entgeltsystem im Krankenhaus. Fallpauschalen-Katalog G-DRG-Version 2018 [Internet]. www.g-drg.de. 2017. https://www.g-drg.de/G-DRG-System_2018/Fallpauschalen-Katalog/Fallpauschalen-Katalog_2018. Accessed 8 Mar 2019.
InEK GmbH—Institut für das Entgeltsystem im Krankenhaus. InEK DRG Browser 2016/2017 [Internet]. 2018. https://download.g-drg.de/DrgBrowser/2018/. Accessed 18 Dec 2018.
Biermann J, Neumann T, Angermann CE, Düngen H-D, Erbel R, Herzog W, et al. Resource use and costs in systolic heart failure according to disease severity: a pooled analysis from the German Competence Network Heart Failure. J Public Health. 2012;20:23–30.
Destatis. Bruttoinlandsprodukt ab 1970 [Internet]. www.destatis.de. 2019. https://www.destatis.de/DE/Themen/Wirtschaft/Volkswirtschaftliche-Gesamtrechnungen-Inlandsprodukt/Tabellen/vermoegensrechnung-bruttoinland-vierteljahresdaten-xls.html.
GKV-Spitzenverband, Verband der Privaten Krankenversicherung, Deutschen Krankenhausgesellschaft. Vereinbarung zum Fallpauschalensystem für Krankenhäuser für das Jahr 2018 [Internet]. 2017. https://www.gkv-spitzenverband.de/media/dokumente/krankenversicherung_1/krankenhaeuser/drg/drg_2018/FPV_2018.pdf. Accessed 14 Jan 2019.
Leyva F, Zegard A, Umar F, Taylor RJ, Acquaye E, Gubran C, et al. Long-term clinical outcomes of cardiac resynchronization therapy with or without defibrillation: impact of the aetiology of cardiomyopathy. EP Europace. 2018;9:1804–12.
Corrao G, Ghirardi A, Ibrahim B, Merlino L, Maggioni AP. Short- and long-term mortality and hospital readmissions among patients with new hospitalization for heart failure: a population-based investigation from Italy. Int J Cardiol. 2015;181:81–7.
Gandjour A, Ostwald DA. Sacubitril/Valsartan (LCZ696): a novel treatment for heart failure and its estimated cost effectiveness, budget impact, and disease burden reduction in Germany. Pharmacoeconomics. 2018;36:1285–96.
Ahlert M, Breyer F, Schwettmann L. How you ask is what you get: framing effects in willingness-to-pay for a QALY. Soc Sci Med. 2016;150:40–8.
National Institute of Health and Care Excellence (NICE). Guide to the methods of technology appraisal 2013. Process and methods [Internet]. www.nice.org.uk. 2013. https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. Accessed 12 Jan 2019.
Barra S, Duehmke R, Providência R, Narayanan K, Reitan C, Roubicek T, et al. Very long-term survival and late sudden cardiac death in cardiac resynchronization therapy patients. European Heart Journal [Internet]. 2019. https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehz238/5482553. Accessed 24 June 2019.
Döring M, Ebert M, Dagres N, Müssigbrodt A, Bode K, Knopp H, et al. Cardiac resynchronization therapy in the ageing population—with or without an implantable defibrillator? Int J Cardiol. 2018;263:48–53.
Desai AS, McMurray JJV, Packer M, Swedberg K, Rouleau JL, Chen F, et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015;36:1990–7.
Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, et al. Declining risk of sudden death in heart failure. N Engl J Med. 2017;377:41–51.
Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–17.
Borne RT, Varosy PD, Masoudi FA. Implantable cardioverter-defibrillator shocks: epidemiology, outcomes, and therapeutic approaches. JAMA Internal Med. 2013;173:859.
Olsen T, Jørgensen OD, Nielsen JC, Thøgersen AM, Philbert BT, Johansen JB. Incidence of device-related infection in 97,750 patients: clinical data from the complete Danish device-cohort (1982–2018). Eur Heart J. 2019;40:1862–9.
Horlbeck FW, Mellert F, Kreuz J, Nickenig G, Schwab JO. Real-world data on the lifespan of implantable cardioverter-defibrillators depending on manufacturers and the amount of ventricular pacing. J Cardiovasc Electrophysiol. 2012;23:1336–42.
Landolina M, Curnis A, Morani G, Vado A, Ammendola E, D’onofrio A, et al. Longevity of implantable cardioverter-defibrillators for cardiac resynchronization therapy in current clinical practice: an analysis according to influencing factors, device generation, and manufacturer. Europace. 2015;17:1251–8.
Gold MR, Padhiar A, Mealing S, Sidhu MK, Tsintzos SI, Abraham WT. Economic value and cost-effectiveness of cardiac resynchronization therapy among patients with mild heart failure. JACC Heart Fail. 2017;5:204–12.
Göhler A, Geisler BP, Manne JM, Kosiborod M, Zhang Z, Weintraub WS, et al. Utility estimates for decision-analytic modeling in chronic heart failure—health states based on New York heart association classes and number of rehospitalizations. Value Health. 2009;12:185–7.
Boston Scientific Corportation. Clinical Summary Contak CD [Internet]. 2011. https://www.bostonscientific.com/content/dam/Manuals/us/current-rev-en/358487-006_US_S.pdf. Accessed 30 Oct 2018.
Tang ASL, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–95.
Abraham WT, Leon AR, Hayes DL. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;9:1845–53.
Cazeau S, Varma C, Haywood GA. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;8:873–80.
Anand IS, Carson P, Galle E, Song R, Boehmer J, Ghali JK, et al. Cardiac Resynchronization Therapy Reduces the Risk of Hospitalizations in Patients With Advanced Heart Failure: results From the Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) Trial. Circulation. 2009;119:969–77.
Schuchert A, Muto C, Maounis T, Frank R, Boulogne E, Polauck A, et al. Lead complications, device infections, and clinical outcomes in the first year after implantation of cardiac resynchronization therapy-defibrillator and cardiac resynchronization therapy-pacemaker. EP Europace. 2013;15:71–6.
Gillis AM, Kerr CR, Philippon F, Newton G, Talajic M, Froeschl M, et al. Impact of cardiac resynchronization therapy on hospitalizations in the resynchronization-defibrillation for ambulatory heart failure trial. Circulation. 2014;129:2021–30.
Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–38.
Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–30.
Acknowledgments
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study concept and the critical revision of the manuscript for important intellectual content. Material preparation, data collection, analysis and production of the first draft were performed by MH. Writing, review editing and interpretation of the results were carried out by F-SF, NE, CE, ND, GH and AK.
Corresponding author
Ethics declarations
Funding
The authors disclose receipt of the following financial support for the research, authorship and/or publication of this article: Funding from the German Federal Joint Committee (Innovationsfonds). Funding code: 01VSF17050.
Conflict of Interest
The authors declare the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: F-S.F.: worked as a consultant for Medtronic. G.H. and N.D.: research grants from Abbott and Boston Scientific to the institution (Heart Center Leipzig) without personal financial benefits. The other authors declare no conflicts of interest.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hadwiger, M., Frielitz, FS., Eisemann, N. et al. Cardiac Resynchronisation Therapy in Patients with Moderate to Severe Heart Failure in Germany: A Cost-Utility Analysis of the Additional Defibrillator. Appl Health Econ Health Policy 19, 57–68 (2021). https://doi.org/10.1007/s40258-020-00571-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-020-00571-y